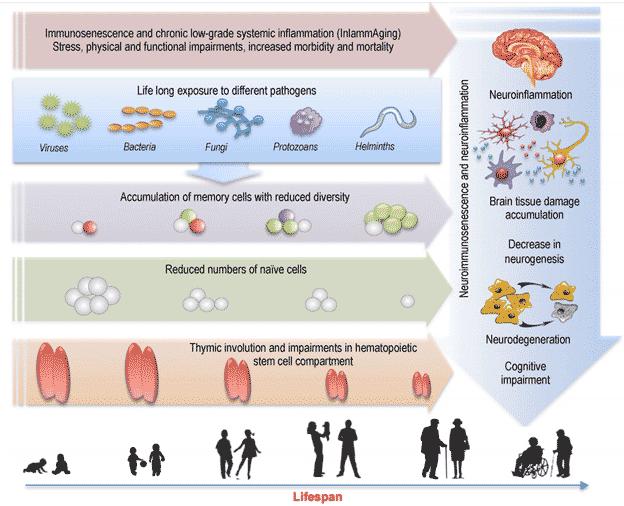
Picture demonstrate of potential mechanisms of immunosenescence and neurosenescence. Immunosenescence or ageing of immune system impact both adaptive and innate immune systems. The actual changes to adaptive immunity are decreased peripheral naive T cells and concomitant accumulation of late-stage differentiated memory T cells with reduced antigen receptor repertoire diversity. This phenomenon results from age-related impairments in the hematopoietic stem cell compartment (which generates few T-cell precursors) in adult and later life, and thymic involution at puberty (which markedly reduces the production of mature T cells from their precursors). Life-long exposure to different pathogens is the major driver of the phenotypic changes in the distribution of T cell subsets over the life course. Aging is characterized by a chronic, low-grade inflammation (termed “inflammaging”) that is a highly significant contributor for morbidity and mortality in the elderly people. Peripheral immunosenescence and inflammaging may promote neuroinflammation by modulating glial cells towards a more active pro-inflammatory state, leading to loss of neuroprotective function, to neuronal dysfunction and accumulation of brain tissue damage. Systemic inflammation may increase the risk for developing cognitive impairments and neurodegeneration.
Source: Di Benedetto et al. / Neuroscience and Biobehavioral Reviews 75 (2017) 114–128
• progressive atrophy of Thymus and all lymphoid tissue (spleen, lymph nodes)
• decrease in the number of T-cells
• increase of immature lymphocyte’s amount
• activation of T-helpers & T-suppressors => stimulation of autoimmune reactions
• decrease in the antigenic activity of lymphocytes
• decrease in anti-tumor resistance
Persistence of chronic infection is associated with lack of adequate phagocytic and digesting possibilities of macrophages, which significantly lengthens period of presentation of foreign antigens to T-lymphocytes and subsequent production of antibodies. Immunosenescence is one of common condition with age-associated dysregulation and dysfunction of immune system characterized by decrease of NK cytoxicity and cytokines production, decline of chemotaxis (neutrophils, monocytes/macrophage and dendritic cells), decline of phagocytosis (macrophages and dendritic cells) and reduction of superoxide production (neutrophils and macrophages) accompanied with thymus involution, shrinkage of T cell repertoire plus chronic inflammatory status.
The shrinkage of the thymus is a biomarker of aging as it promotes appearance of chronic infections, persistence and recurrence of infections, occurrence of cancer and autoimmune diseases increases, depression also low down immunity and many drugs are immunosuppressive.
Thymus extract alone showed a therapeutic effect on:
Long term immunotherapy of patients with immunodeficient conditions, bone marrow failure, autoimmune disorders, chronic skin diseases, recurrent viral and bacterial infectious diseases and some oncological disorders resulted in the most of the observed patients, in improvement of symptoms and features of the disease and in parallel regulation of disturbed immune parameters (Skotnicki, 1989). It is suggested that thymus would support, modify and enrich future treatment protocols, especially in patients with immunodeficiency-related or chronic immune-mediated diseases.

