STF are stimulating the activity of the Natural Killer (NK) Cells, they control viral infections and tumors. The main duty of killer cells is to identify and destroy the body’s own cells in which something is broken: they kill tumor cells and cells infected with viruses (and also, possibly, with other alien agents). NKs recognize certain structures of high molecular weight glycoproteins that are expressed on the membrane of virus-infected cells. Recognition of the target cell and convergence with it occurs at the expense of NK receptors. As a result, NK are activated, and the contents of the granules are released into the extracellular space. Perhaps the main role here belongs to perforin (cytolysin), which has some structural similarity with the component of complement C9 (antibodies to perforin suppress extracellular destruction). Perforin is embedded in the membrane of the target cell and forms transmembrane pores, which leads to cell death, as the contents of the cell flow out through these pores. In addition, NK granules contain two serine protein kinases that can function as cytotoxic factors, but their role in NK-dependent lysis is not entirely clear. Chondroitin A sulfate, proteoglycan resistant to protein kinases, is also found in NK and can protect these cells from autolysis. When the target is recognized, NK cells are capable of both “positive” and “negative” recognition.
Unlike T-killer cells, NK cells carry a cytotoxicity receptor (KIR killer inhibitory receptor). When negative recognition interacting with MHC class I molecules on the target cell, these receptors give the infected cell a signal to inhibit its cytotoxic activity. Positive recognition occurs when there is no expression of MHC molecules on target cells, and the interaction of NK cells with infected cells occurs with the participation of their own (NK cells) specific receptors, in particular CD2 and CD69, or antibodies with which they bind through the receptor for Fc (CD16). The binding of NK to antibodies that form immune complexes with antigens on the surface of target cells is interpreted as a manifestation of killer cell activity, or antibody-dependent cellular cytotoxicity. For example, herpes viruses try to avoid T-killer recognition by suppressing the expression of MHC class I molecules of infected cells surface; however, in this case, NK cells recognize the virus. The set of cells exposed to the lytic action of NK is quite wide. This is a series of virus-infected and tumor cells; cells on the surface of which cytophil antibodies are represented; embryonic cells.
These cells were called natural killers because, according to early ideas, they did not require activation to destroy cells that did not carry markers of the main type I histocompatibility complex. The main function of NK is to destroy the cells of the body that do not carry MHC1 on their surface and are thus inaccessible to the action of the main component of antiviral immunity, T-killer cells. A decrease in the number of MHC1 on the cell surface can be a consequence of the transformation of the cell into a cancer cell or the action of viruses such as the papillomavirus and HIV. Although NK morphologically resembles lymphocytes or lymphoblasts, their histogenetic association with T or B lymphocytes has not been established. Probably, NK belong to an independent line of differentiation, although at the earliest stages of development they have a common precursor with lymphocytes. Unlike lymphocytes, NKs do not have antigen recognition receptors, do not increase quantitatively after interacting with an alien (eg, viral) antigen, and are not in demand for the formation of immunological memory. Moreover, their activity increases under the influence of T-cell cytokines and, first of all, interferon-gamma.
The ability of NK to recognize “its” and “alien” on the cells is determined by surface receptors. NK has a complex system of receptors that recognize molecules of the body’s own cells. In addition, NKs have many receptors for stress-induced cell ligands that indicate cell damage. These receptors include natural cytotoxicity receptors (NCRs), NKG2D, which activate the cytotoxic functions of NK. Therefore, cytotoxic T-cells (T-killers) and NK-cells can be considered as two complementary tools of immunity against viral infection of tissues. NK lymphocytes play an important role in antiviral and antitumor immunity and are involved in transplant rejection. A decrease in the cytotoxic activity of NK lymphocytes is detected in many diseases, including malignant tumors, and the absence is extremely rare. Natural (normal) killer cells (NK) are a population of lymphoid cells devoid of signs of T-lymphocytes or B-lymphocytes.
Initially, natural killers were detected by their ability to kill lymphoid tumor cells in vitro without any prior priming. Later it became known that these cells are involved in non-specific protection against some viral and bacterial intracellular pathogens. Active NK cells appear already two days after infection of the host virus. Their activity increases 20-100 times under the influence of cytokines IF-alpha and IF-beta, as well as IL-12, produced by macrophages in the early period of infection.
IL-12, together with TNF-alpha, also formed by macrophages, provides for the active secretion of IF-gamma by NK-cells. The secretion of IF-gamma also increases the functional activity of NK cells, which creates conditions for early protection against some infections until T-cells producing the same cytokine react. Mice deficient in T or B cells are quite resistant to the intracellular bacterial pathogen Listeria monocytogenes. However, when there is a shortage of infected mice by NK cells, TNF-alpha or IF-gamma, an infection develops, which destroys animals even before the inclusion of primed T-cells in the response.
Normal killer cells (NK cells) in humans constitute approximately 5% of peripheral blood lymphocytes. The NK cells are an exceptional type of lymphocyte as they need no priming or training to function as the T- and B-lymphocytes do. They recognize foreignness and rapidly destroy them. NK cells are large granular lymphocytes and known to differentiate and mature in the bone marrow, lymph node, spleen, tonsils and thymus where they then enter into the circulation.
This plateau is due to the NK cells action killing virally-infected cells and stopping the replication of the virus. Obviously the earlier and effective NK cell function is critical and determines the outcome and duration of an illness. STF has been reported as a modulator of the immune response, which up-regulated synthesis of molecules such as IL-2, the activation and chemotaxis of macrophages and natural killer cells.
Supplementing immune function with STF activate NK cell much earlier. So the recognition and destruction possible before it becomes recognized as a disease. Prevention is available as STF acts within 24 hours.
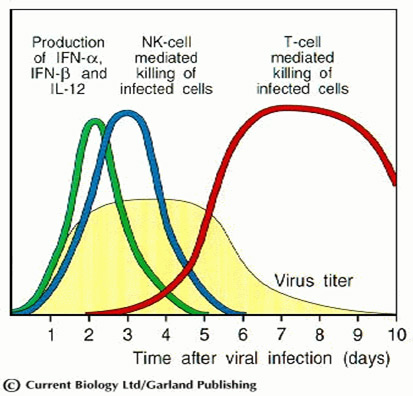
The immune responses to a viral infection on days.

