The positive responses are confirmed through various tests such as delayed hypersensitivity test on skin, response to alloantigen, specific and non-specific mitogen, type of T cell, NK cell activity, cytokines activity (Krishnaveni, 2013) Using Transfer Factor in case of Osteosarcoma increased cell mediated cytotoxicity (Fudenberg, 1976) and in case of Varicella with acute leukemia in children and Herpes simplex virus research demonstrated the improvement in T cell function (Russell, 1980) (Russell, 1976) (Viza, 1986). Increased C3 level returned to normal, no new infection, absence of eczema was observed in patients with Wiskotte -Aldrich syndrome (Levin, 1970) and in case of Glioma – reduced size of tumor, and increases CD2þ, CD4þ, CD8þ and NK cell counts, apoptotic tumor cells (Pineda, 2005;). Patient with Prostate cancer with transfer factor showed higher survival rates among the same group (Pizza, 1996) and HIV cases -increased levels of helper T cells and cytotoxic T cells (Granitov, 2002). Longer survival was also demonstrated in Lung cancer patients (Pilotti, 1996). Transfer factor in cases of Hepatitis C – stimulates Th1, which helps in clearing of viral particles (Milich, 1998) (Tsai SL, 1997). Chronic mucocutaneous candidiasis patients showed restored cellular immunity (Masi, 1996).
- STF lack of viable cells that play a role in graft versus host reaction, not immunogenic, contain no histocompatibility antigens.
- T helper cells are active in fighting against the pathogen, thereby stimulating the production of new helper T cells, Natural killer cells, macrophages, cytotoxic T cells.
- To specify the target STF influence the expression of antigen receptors on cells. Increased Th1 in turn repress the production of Th2 and its cytokines like IL-4, IL-5, IL-6, and IL-13.
- IMPs in form of transfer factor promotes an immune response mediated by cytokines that indirectly induce the proliferation of hematopoietic progenitor cells in bone marrow, as reported used pig-DLE in rats after radiotherapy(Vacek A, 2005).
Therefore, transfer factor gives an attractive option to complement chemotherapy, which might enhance the immune system after disturbances resulting from the side effects of chemotherapy (Atzpodien J, 2003) (Ojeda MO, 2005) (Kirkpatrick CH, 1985). STF is effective in improving cellular immunity and in regulating the production of different cytokines involved in tumor progression.
In other reports, the administration of IMPs (STF, TF or DLE) directly to the experimental glioma showed the reduction of tumor size and increase of CD2+, CD4+, CD8+ and NK cell counts in rats with glioblastoma multiforme. It was also increased the percentage of apoptotic tumor cells and the percentage of tumor tissue expressing Th1 cytokines. Researchers observed an additive antitumor effect when transfer factor was combined with chemotherapy. (Pineda, Interstitial transfer factor as adjuvant immunotherapy for experimental glioma, 2005)
The main benefit of IMPs in form of STF as immunotherapeutic agents is the possibility to stimulate a rapid immune response against the pathogen which was documented within 24 h and thus reduce the time for the patient immune response near to 9–13 days (Arnaudov, 2017).
Another study showed the decrease of inflammatory infiltrates, morphological changes to similar of normal prostate, and the prostatein decreased to the basal levels in a group treated with DLE. DLE treatment produced a decreased expression of the cell surface marker CD45 and the proinflammatory cytokines TNF-α, IFN-γ, IL-6, and IL-17. The study conclusion: DLE is able to modulate the inflammatory response in Experimental Autoimmune Prostatitis (Pérez-Alvarado, 2017)
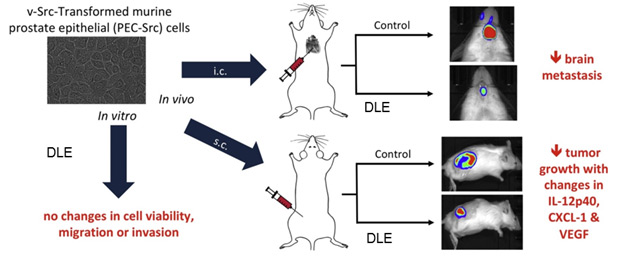
Prostate cancer is the second most often diagnosed cancer in men worldwide. Was employed v-Src-transformed murine prostate epithelial (PEC-Src) cells, which recapitulate the transcriptional profiles in human PCa, can be grown in immunocompetent mice, and consistently form bone and brain metastases. In vitro, DLEs did not induce cytotoxicity nor alterations in migration /invasion of PEC-Src cells. In vivo, DLEs reduced metastatic dissemination after intracardiac injection of PEC-Src and inhibited tumour growth of subcutaneous isotransplants. The antineoplastic effect of DLEs correlated with changes in tumour infiltration, increased serum concentrations of IL-12 and CXCL1, and reduced levels of VEGF (vascular endothelial growth factor). The results propose that the antineoplastic effect produced by DLEs is due to its immunomodulatory effect and not by a direct effect on cancer cells, and indicate that DLEs could be beneficial as adjuvant therapy in PCa patients. (A.Velasco-Velázquez, 2018)
PET-CT was used to evaluate both groups and observed that in the group with DLE as adjuvant the regression of metastatic lesions in diverse anatomic locations was obtained in less time than in the control group (without DLE) (HUMBERTO, 2010).
Source: Establishment and evaluation of a new highly metastatic tumor cell line 5a-D-Luc-ZsGreen expressing both luciferase and green fluorescent protein, published at International Journal of Oncology.
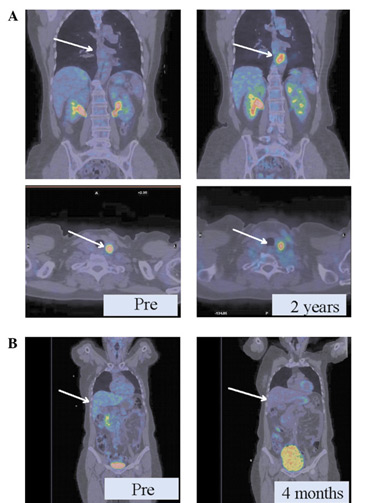
As shown in Pic.A, patients with metastatic breast cancer without adjuvant therapy had persistent thyroid lesions and a new lesion around the aorta (2 cm) after 2 years of chemotherapy.
In another case (Pic.B), retroperitoneal retrohepatic metastases exhibited a partial regression after only 4 months of receiving DLE treatment and 5 cycles of chemotherapy.
Immune modulating peptides (STF, DLE, TF) as an adjuvant of chemotherapy has been associated with tumor regression and temporary stabilization in several types of cancer (Meier, 1977):
- such as breast cancer, nasopharyngeal carcinoma. Goldenberg GJ et al. In vivo and in vitro studies of immunotherapy of nasopharyngeal carcinoma with transfer factor (Goldenberg, 1976)
- metastatic renal carcinoma, Cancer and complementary and alternative medicine in Italy: personal observations and historical considerations (Moss, 2004)
- prostate cancer A preliminary report on the use of transfer factor for treating stage D3 hormone-unresponsive metastatic prostate cancer (Pizza G. , 1996).
- and others like patients with osteosarcoma received adjuvant treatment with specific transfer factor (Rodriguez-Flores, 2009).
- In control group after several cycles of chemotherapy the quantaty of lymphocytes decreased to below the reference range. Contrary, in patients with DLE as an adjuvant, the CD4+and CD8+ and B lymphocytes stayed within the median value of the reference range and also NK cells amount increased after chemotherapy. In conclusion, DLE treatment supports considerable immunological recovery after numerous chemotherapy, improving the treatment compliance and quality of life throughout chemotherapy.
- No myelosuppression in lymphoid populations was shown in patients receiving the DLE treatment, while in patients undergoing chemotherapy without DLE the absolute numbers of CD4+, CD8+and B lymphocytes were reduced compared to the reference range values (HUMBERTO, 2010).
- Considerable increase in the numbers of NK cells (P<0.05) and B lymphocytes (P<0.05) was documented 1 month after the completion of chemotherapy in patients receiving DLE as an adjuvant.
- IL-3 levels dropped by 80% in the control group without The group with DLE as an adjuvant during chemotherapy demostrated decrease by 34% only.
- IL-7 levels elevated in the group that received DLE as an adjuvant (11%) prior to chemotherapy in combination with DLE and decreased by 30% in the control group during chemotherapy as compared to before chemotherapy.

